
WATER ADJUSTMENT
Share
We do not want to claim that water adjustment is an uncomplicated subject, on the contrary, it is an incredibly comprehensive and complex subject. We recommend reading the book "Water" by John Palmer who also wrote the eminent book "How to brew" if you want to delve deeper into the subject and understand how this works on a chemical and biological level.
But! We'll skip that in this article and go straight into what it takes to get started with simple water adjustments that yield noticeably good results.
1. WATER PROFILE
The first thing you need is a water profile for your water.
For those with municipal water, this is a simple matter, since an analysis report should always be available to obtain from the water utility , or those responsible for water production .
They are usually available for download from the municipality's website. You can also email the municipality's customer service and request a water report/analysis report for your drinking water.
We will return to how to add the water profile to the construction program.
Water adjustment is the addition of salts / minerals / acids to change the amount of ions in the brew water, adjust the pH, as well as add calcium that the yeast needs. These are stated in milligrams per liter (mg/L) or in parts per million (PPM) , which are two units to indicate the same thing when assuming that one liter weighs one million milligrams.

2. SALTS
In the water profile you will find (calcium) chloride , (calcium) sulfate and magnesium, which are among the salts we use to adjust the brewing water. We also use calcium carbonate and sodium bicarbonate .
The water we get at home from the tap is treated in many ways through filtration, chlorination, UV light and added carbon dioxide so that the PH value is usually around 8, which gives slightly alkaline water. When we add malt to the brewing water, the pH will be pulled down as the malt is more acidic than the water, but usually the malt alone is not enough to reach the desired pH of 5.2-5.4 . We therefore add salts and acids that help us to achieve a better result.
In addition to changing the pH, the salts have other properties such as adding calcium and magnesium, which are necessary for a good fermentation process, better yield of the malt and improved flocculation of proteins, which results in clearer beer faster.
Below we will list 5 salts that are most commonly used in water adjustment.
The first two salts lower the pH, while the next two increase the pH of the mash. The last one has little to do with pH.
Calcium sulfate is referred to as CaSO4 or Gypsum and pulls the pH down.
Calcium sulfate enhances hop character and balances bitterness in the beer . It is therefore used in hop-driven beers such as classic IPA and APA. Calcium sulfate will also increase the amount of calcium in the water, which provides the benefits we have described in the section above.
Calcium chloride is referred to as CaCl2 or " road salt " and lowers pH . Calcium chloride provides increased mouthfeel, a sweet and smooth flavor profile and is often used in malty beers but is also an important ingredient for those who want to brew a true NEIPA . If you have achieved the desired amount of calcium in the water but want more chloride for increased mouthfeel, you can use a little table salt (sodium chloride - NaCl). Calcium chloride, just like calcium sulfate, provides increased calcium in the brew water.
Calcium carbonate is referred to as CaCO₃ or lime and raises the pH .
This is acid neutralizing and will give the water a higher alkalinity , which means that more acid can be added without the pH being lowered too far .
Calcium carbonate is therefore used in brewing darker beers as the dark malt is much more acidic than base malt and significantly lowers the pH . Without acid neutralizing salts, the beer will be thinner, more acidic/sour and more unbalanced. Not as velvety as you would like.
Calcium carbonate is not easily soluble in tap water due to its high pH, and is therefore added directly to the mash along with the malt as it dissolves more easily in a more acidic environment.
Sodium bicarbonate is referred to as NaHCO3 or Natron which raises the pH.
Like calcium carbonate, sodium bicarbonate is an antacid, but it is more soluble in water and provides a higher buffering capacity to the water than calcium carbonate does.
Magnesium sulfate refers to MgSO4. Yeast needs magnesium and it is therefore normal to add a little magnesium sulfate for the sake of yeast . Magnesium sulfate also increases the hardness of the water, which can affect beers such as porter and stout . Excessive use will give the beer a sour/bitter taste. Recommended dosage below 30mg/liter.
The salts are often used in combinations to achieve different results.
For example, in an IPA, you would use "large amounts" of calcium sulfate to emphasize hops and bitterness, often along with calcium chloride. This would lower the pH too much and you would then use calcium carbonate and/or sodium bicarbonate to avoid the pH being pulled too far down.
Similarly, calcium chloride can be added to dark beers to increase sweetness and mouthfeel when sodium bicarbonate is added at the same time, which neutralizes the acid from the dark malt and prevents the calcium from calcium chloride from significantly lowering the pH.

3. ACIDS
The function of acid in brewing is to lower the pH of the brewing water.
The primary acids used in brewing are lactic acid and phosphoric acid. These are two relatively weak acids that are both food-grade.
Another useful product is acid malt, which is malted barley bathed in lactic acid. 1% acid malt of the total malt quantity lowers the pH by approx. 0.1. Acid malt is also compatible with the Reinheitsgebot for those who believe it is important to brew with nothing but water, malt, yeast and hops.
Acid is used to reduce the pH of the brewing water to reach a pH of 5.2-5.4, either in combination with the salts above or alone if it is desired not to add more calcium.

4. pH meter
With water adjustment, we have the opportunity to adjust the pH, which is important for optimal mash.
It is optimal to have a pH of:
- 5.2 for light fresh beers
- 5.4 for darker and fuller beer .
There are therefore big differences within a tiny range of a few decimal places and you have to be careful to hit where you want.
5. WEIGHT
A sensitive scale is necessary, as it involves a few grams per batch of 20-25 liters. A pipette or measuring spoon is nice to have to measure out the right amount of acid.
6. BREWING PROGRAM
A brewing program such as Brewfather , Beersmith , Promash etc. is a good guide in terms of how much salts and acid is needed for the different batches.
In the guide further down the page, we show how to do it in Brewfather, which has a water adjustment module that is easy to understand.
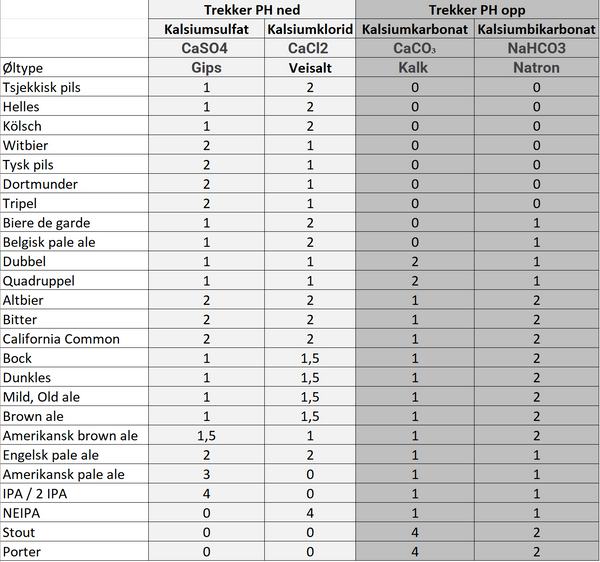
Here we have pasted a table prepared by Gahr Smith Gahrsen , brewmaster at 7 Fjell Bryggeri in Bergen . Gahrsen has based his work on notes from the American homebrewing legend John Palmer, who has written the books " How to brew " and " Water ".
The drinking water we have access to in this country is most often from surface water sources and is therefore soft and low in minerals . There are small differences from city to city and the table below can therefore be used as a good starting point.
Contact your local water company for more details about your specific water.
CaSO4 = Calcium sulfate
CaCI2 = Calcium chloride
CaCO₃ = Calcium carbonate
NaHCO3 = Calcium bicarbonate
All additives are grams per 10 liters.
The appointments that attract:
-
pH down should be added once to the mash and once to the rinse water.
-
pH up should only be added to mash.
Water adjustment in Brewfather
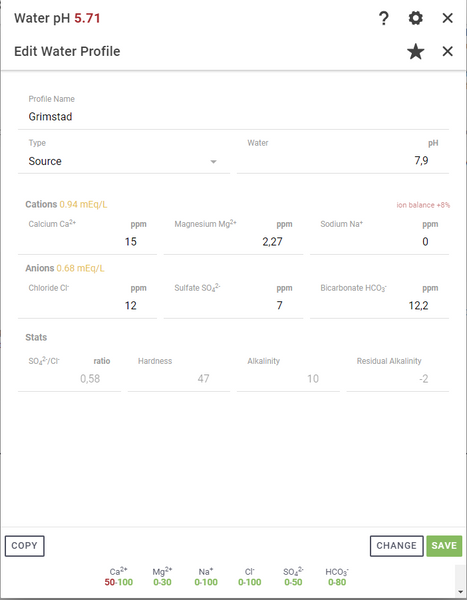
To add a water profile in Brewfather go to Profiles -> Water -> Press the “+Add Profile” button
Once we have entered the values from Grimstad's drinking water, we get a profile that looks like this:
Then we go to the recipe module, retrieve a recipe, scroll down to Water and press the “CALC” button.
When we enter the “Edit water profile” module, check that the amounts of water are in order with the expected values. Then under Source, press CHANGE to select the drinking water profile you just created.
Skip Target Profile if you don't want to mimic a specific water.
Under Style, choose a type that is closest to the style you want to emulate. Once you have made a choice, you will find out what values you are recommended to stay within.
In this guide we focus on calcium sulfate and calcium chloride . In some styles it may be appropriate to use Calcium Carbonate (CaCO3, Lime), Magnesium Sulphate (MgSO4, Epson Salt) and for example in Gose it is common to use Sodium Chloride (NaCl, table salt).
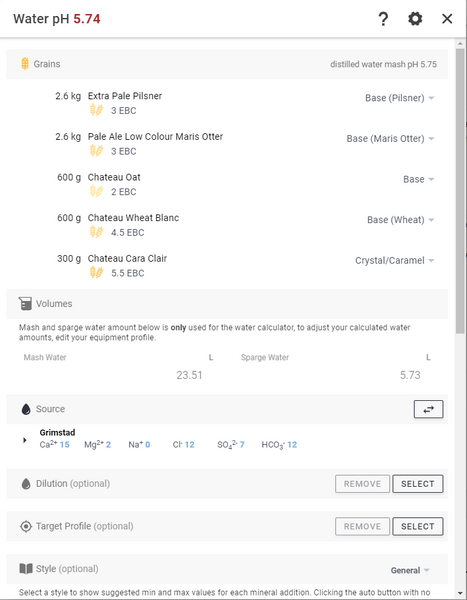
In the example here is a water profile that works well for a NEIPA . If you want to increase the smoothness and fullness in the mouthfeel, you can reduce the amount of calcium sulfate and increase the amount of calcium chloride, or even drop the calcium sulfate altogether.
If you find that the calcium value is too high , you can add table salt which increases chloride without affecting calcium . But do not use too much as this can give the beer a salty taste. This is a matter of taste and preference and there is no definitive answer, you have to try it yourself.
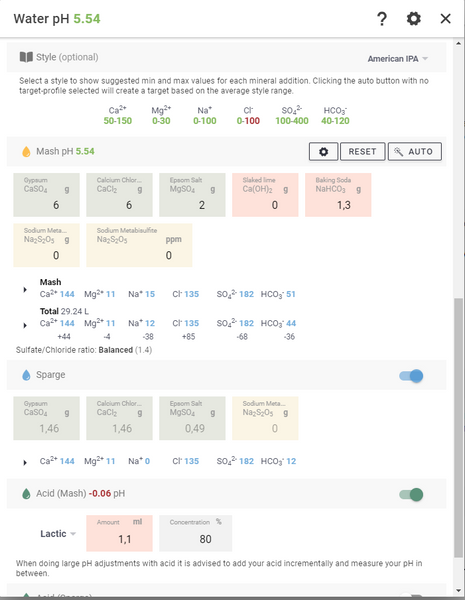
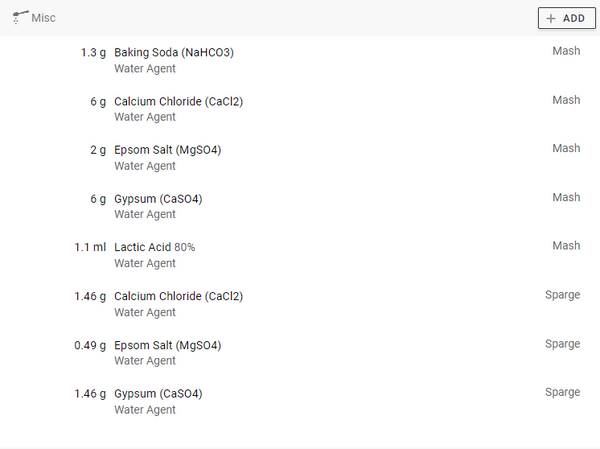
Turn on the "Sparge" option to increase the amount of salts that can be added directly to the rinse water. This is to prevent the amount from becoming too concentrated in the mash.
To adjust the pH, turn on the switch next to " Acid (Mash)", select the type of acid you have available and what concentration it is. Increase the "Amount" until the "Mash pH" in the header is down to the desired pH. Make sure you have already entered the amount of salts, as these also affect the pH.
It is highly recommended to activate the “ Acid (sparge)” rinse water. It is critical to lower the PH of the rinse water to avoid astringency and tannins in the beer. With a little lactic acid in the rinse water, these potential problems are gone.
Don't forget to adjust the concentration. Our lactic acid is 80% and phosphoric acid is 10%. When you press "Save adjustments to recipe" the amount of salts and acids will be added to the recipe.
Some would argue that using a pH meter is critical, but we would like to argue that it works fine either way. Today's brewing programs are so good that the given values will be good enough to just throw the salts in the pot and go.
In our test brews, the actual value has been very close to the theoretical one. Sometimes a little above, sometimes a little below.
